Multimedia Gallery
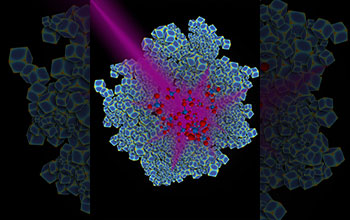
Beam of ultraviolet light hitting swarm of rhodium nanocubes
A beam of ultraviolet light hits a swarm of rhodium nanocubes. Duke University researchers have engineered rhodium nanoparticles (blue) that can harness the energy in ultraviolet light and use it to catalyze the conversion of carbon dioxide to methane, a key building block for many types of fuels.
More about this image
A team of researchers at Duke University has developed tiny nanoparticles that help convert carbon dioxide (CO2) into methane using only ultraviolet light as an energy source. The team hopes to next develop a version that would run on natural sunlight instead of ultraviolet, a potential boon to alternative energy.
Chemists have long sought an efficient, light-driven catalyst to power this reaction, which could help reduce the growing levels of (CO2) in the atmosphere by converting it into methane, a key building block for many types of fuels.
Not only are the rhodium nanoparticles made more efficient when illuminated by light, they have the advantage of strongly favoring the formation of methane rather than an equal mix of methane and undesirable side-products like carbon monoxide. This strong "selectivity" of the light-driven catalysis may also extend to other important chemical reactions, the researchers say.
"The fact that you can use light to influence a specific reaction pathway is very exciting," said Jie Liu, the George B. Geller professor of chemistry at Duke. "This discovery will really advance the understanding of catalysis."
The research was supported in part by a grant from the National Science Foundation to explore the fundamentals of plasmonic photocatalysis (grant CHE 1565657).
To learn more about this research, see the NSF News From the Field story Light-driven reaction converts carbon dioxide into fuel. (Date image taken: 2015-2017; date originally posted to NSF Multimedia Gallery: March 29, 2017)
Credit: Chad Scales
Images and other media in the National Science Foundation Multimedia Gallery are available for use in print and electronic material by NSF employees, members of the media, university staff, teachers and the general public. All media in the gallery are intended for personal, educational and nonprofit/non-commercial use only.
Images credited to the National Science Foundation, a federal agency, are in the public domain. The images were created by employees of the United States Government as part of their official duties or prepared by contractors as "works for hire" for NSF. You may freely use NSF-credited images and, at your discretion, credit NSF with a "Courtesy: National Science Foundation" notation.
Additional information about general usage can be found in Conditions.
Also Available:
Download the high-resolution JPG version of the image. (840.1 KB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.