Multimedia Gallery
Water-air Interface of Acidic Solutions
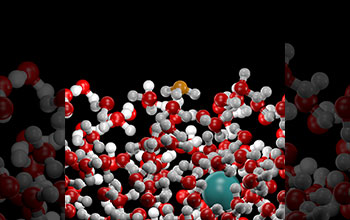
An image from a molecular dynamics computer simulation of the water-air interface of acidic solutions. The acid molecule (hydronium ion, shown in orange) has a counterintuitive tendency to prefer the interface instead of remaining buried deeper in the liquid region.
More about this Image
This behavior arises primarily from the molecular asymmetry on the ion, which leads to its preference for the interface. This result has highly significant implications for atmospheric chemistry, acid rain pollution, energy production by living organisms and the efficient utilization of hydrogen as a fuel in fuel cell technology.
This research was supported by National Science Foundation grant CHE 0317132. (Date of Image: Aug. 15, 2005)
Credit: Image created by Matt Petersen from the research group of Prof. G. A. Voth, University of Utah
Images and other media in the National Science Foundation Multimedia Gallery are available for use in print and electronic material by NSF employees, members of the media, university staff, teachers and the general public. All media in the gallery are intended for personal, educational and nonprofit/non-commercial use only.
Images credited to the National Science Foundation, a federal agency, are in the public domain. The images were created by employees of the United States Government as part of their official duties or prepared by contractors as "works for hire" for NSF. You may freely use NSF-credited images and, at your discretion, credit NSF with a "Courtesy: National Science Foundation" notation.
Additional information about general usage can be found in Conditions.
Also Available:
Download the high-resolution JPG version of the image. (532.1 KB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.