Multimedia Gallery
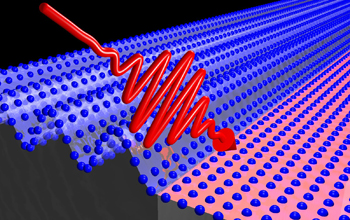
Vanadium dioxide crystal lattice
A vanadium dioxide crystal lattice. The vibrational properties of atoms in a lattice are determined by the surrounding electron clouds (blue wave front). These oscillations can be suppressed by a sufficiently strong absorption of light (red) that drives a phase transition.
More about this image
An international team of physicists, led by Richard Haglund, a physics professor at Vanderbilt University, developed a method for taking ultra-fast "sonograms" of materials undergoing phase transitions. Phase transitions are structural changes that produce dramatic changes in a material's physical properties -- such as the melting of candle wax before it burns and dissolving sugar in water -- and play a critical role both in nature and in industrial processes ranging from the making of steel to chip fabrication.
The method can track the structural changes that take place within solid materials in trillionth-of-a-second intervals and sheds new light on the manner in which vanadium dioxide, the material that undergoes the fastest phase transition known, shifts between its transparent and reflective phases.
The research was funded in part by the National Science Foundation (grant ECCS 08-01985). To learn more, see the Vanderbilt news story Ultrafast sonograms shed new light on rapid phase transitions. (Date of Image: March 2012)
Credit: Julia Stähler, Fritz Haber Institute of the Max Planck Society
See other images like this on your iPhone or iPad download NSF Science Zone on the Apple App Store.
Images and other media in the National Science Foundation Multimedia Gallery are available for use in print and electronic material by NSF employees, members of the media, university staff, teachers and the general public. All media in the gallery are intended for personal, educational and nonprofit/non-commercial use only.
Images credited to the National Science Foundation, a federal agency, are in the public domain. The images were created by employees of the United States Government as part of their official duties or prepared by contractors as "works for hire" for NSF. You may freely use NSF-credited images and, at your discretion, credit NSF with a "Courtesy: National Science Foundation" notation.
Additional information about general usage can be found in Conditions.
Also Available:
Download the high-resolution JPG version of the image. (1.5 MB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.